
Commissioning and qualification
Ensure facilities, systems, and equipment are designed, installed, tested, and operated per requirements — as efficiently as possible.


Solutions for Contract Manufacturing
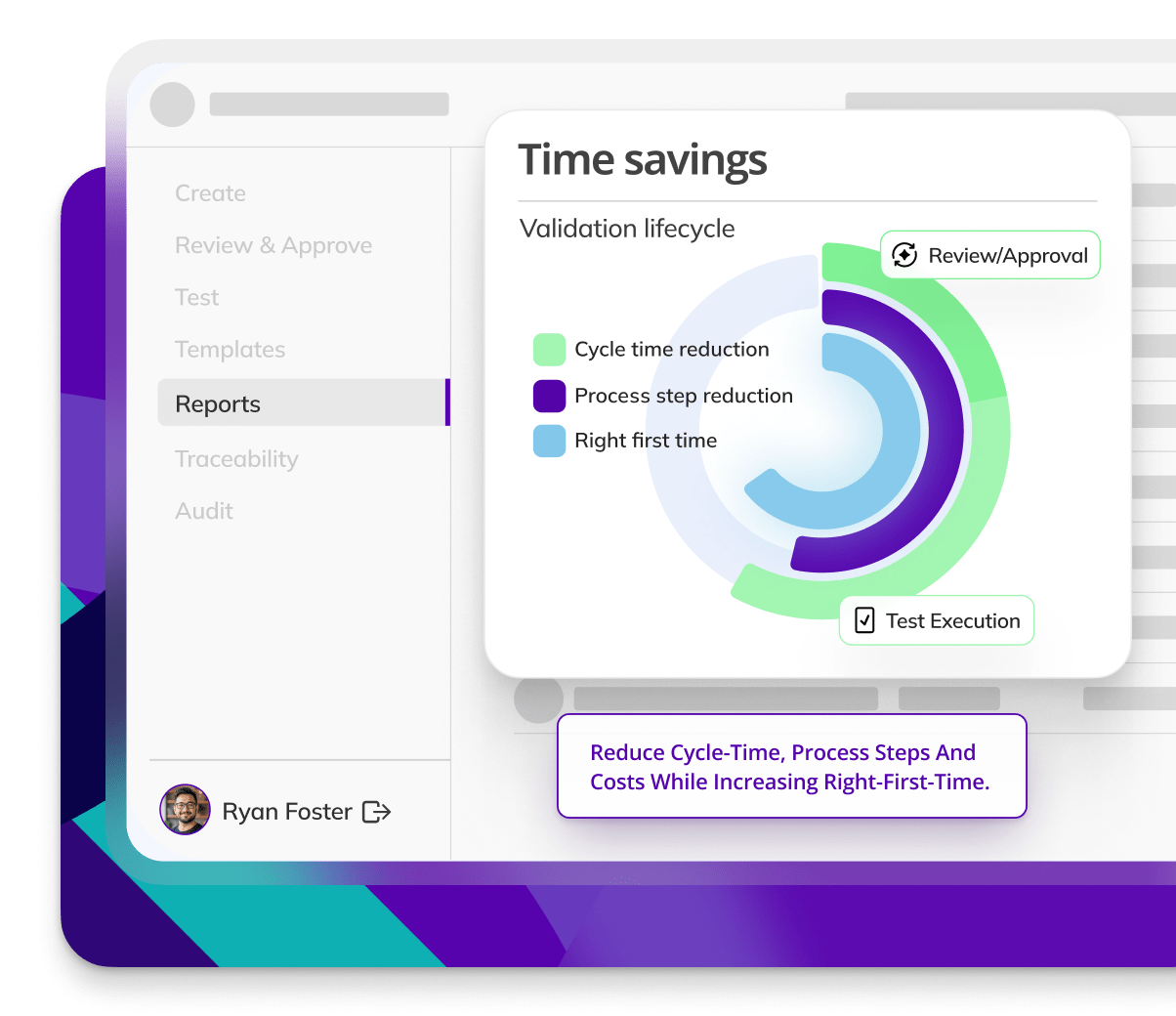
Accelerate time to market — Kneat Gx cuts cycle times by 50% and validation steps by 46%, helping CDMOs release products faster.
Talk to an expertTrusted By










Common Validation Challenges
For CDMOs, managing validation risk is key. Our software helps automate your operation, reduce manual effort, and streamline validation. Easily document every protocol, record each signature, and provide evidence for final approval. Ensure accuracy of data, detect issues during review and inspection, and support assurance of product quality for the market.
Challenges
Meeting diverse client needs, handling technology transfers, and managing a wide variety of regulatory requirements can be challenging. Maintaining quality agreements and ensuring process integrity, while navigating the complexity of multi-product validation are essential for success.
Contract manufacturers serve multiple clients, each with unique validation protocols and standards, necessitating flexible and client-specific validation approaches. Kneat enables quick and easy replication of client protocol and test-case templates.
Validating processes as part of tech transfer between client and CMO demands a deep understanding and precise replication of processes to ensure consistency and maintain product integrity. Kneat centralizes data, creating a clear, accessible single source of truth.
Manufacturing pharmaceuticals and medical devices requires compliance with various regulatory standards, adding complexity to validation process while ensuring quality across diverse products. Kneat automatically tracks compliance across varying standards.
Ensuring that all validation activities align with the quality agreements established with clients is essential to meet contractual obligations and regulatory expectations. Kneat ensures process adherence with set workflows.
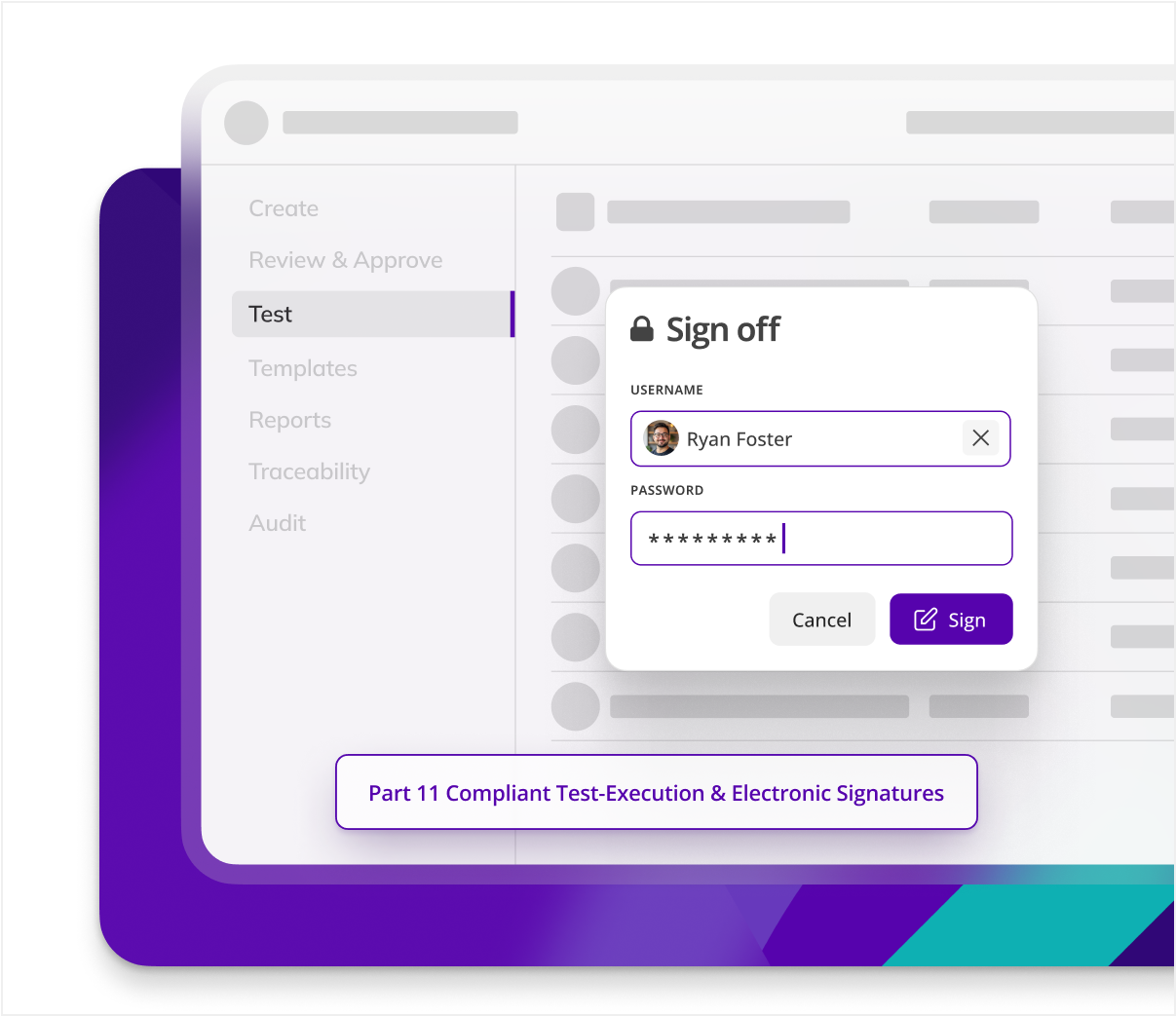
THE SOLUTION
Kneat is designed for and trusted by the Pharma industry. Digitalize and manage any validation process your way, in one easy to use, end to end solution.
BENEFITS
Kneat streamlines client onboarding and tech transfers, accelerating validation to minimize delays between contracting and production. With Kneat, manufacturers achieve faster, more reliable, and fully transparent validation, ensuring seamless handovers and rapid time to value.

We are faster and more efficient with Kneat. Test cases are getting completed faster. As a Project Manager, I like that everything is in one place and easily accessible.
IT Project Manager, Leading Contract Research Company
Download case studyDigitalize validation your way, with the validation platform trusted by the world’s leading life sciences companies.
Talk to an expertCustomer Success
A top 10 biotech leader
The customer, a rapidly expanding biotechnology company with over 45,000 employees achieved an 88% time saving on URS, reducing the cycle time from 2 months...
use cases

Merck Sharp & Dohme
MSD digitized seven validation processes globally, reducing cycle times by 50%, process steps by 46%, and replacing three QMS systems.
use cases

ElevateBio
ElevateBio reduced cycle times by 50%, accelerated manufacturing changeovers, and delivered top-tier quality assurance with Kneat.
use cases

Top 10 Pharma Leader
Pharma leader standardized C&Q with Kneat, achieving 20–25% cost savings and 30–40% reduction in validation cycle time.
use cases

The customer, a rapidly expanding biotechnology company with over 45,000...
MSD digitized seven validation processes globally, reducing cycle times by...
ElevateBio reduced cycle times by 50%, accelerated manufacturing changeovers, and...
Pharma leader standardized C&Q with Kneat, achieving 20–25% cost savings...

Ensure facilities, systems, and equipment are designed, installed, tested, and operated per requirements — as efficiently as possible.

Maintain a state of continuous compliance and documentation accuracy to ensure preparedness for regulatory inspections and all forms of audits.

Ensure batch records are complete, accurate, and compliant. Providing traceability and supporting product quality assurance.
Industry Solutions
We are built for biotechnology, pharmaceuticals, medical devices, and consumer health.

Our expert team and industry-leading solution help pharma quality assurance and validation teams overcome compliance and operational challenges every day.

Get more products to market faster without compromising quality. Improve the efficiency of the entire validation lifecycle for all your consumer health products.

Overcome complexity across process, analytical method, equipment, cleaning, computer system, assay, sterilization, and facility validations.

Transform the efficiency and compliance of design, process, software, cleaning, equipment, packaging, sterilization, and test method validations.





Kneat experts talk about the most significant updates to GMP guidelines in years
14:00 (60 minutes)

Get a practical overview of ISPE’s Digital Validation Guide by Kneat’s experts.

G2 has ranked Kneat best pharma and biotech software in Grid report, rating Kneat as the top pick in satisfaction....